
News
Research Interests
Metals are considered by many as toxic to humans and therefore unsuitable to be used in medicine. In the Frei Lab we are working towards changing this perception by developing novel metal-based antimicrobials to fight the global antibiotic resistance crisis.
Why do we need new antibiotics? Why are metal compounds a possible solution? Find out in this video produced in collaboration with the University of Bern
-
In the Frei Lab we are exploring new classes of metal complexes for their antimicrobial potential.
-
One focus is the elucidation of the Mode of Action of these compounds. Understanding this will help us design even better metalloantibiotics
-
We are exploring metal-based photosensitizers for aPDT and investigating their potential to avoid resistance in bacteria
-
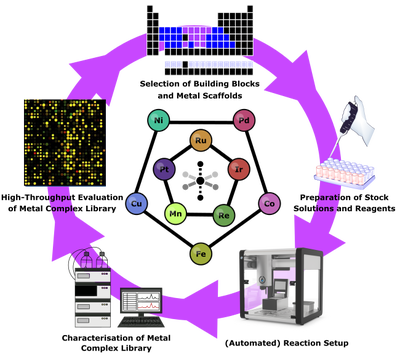
To efficiently explore the vast metal-complex space we are adapting machine-learning based solutions to coordination compounds
The Antimicrobial Resistance (AMR) Crisis
The WHO warns that “without urgent, coordinated action“, 10 million people will die each year due to antimicrobial resistance (AMR) by 2050. If this becomes reality, AMR would surpass cancer as the leading cause of death in the western world and become deadlier than the current COVID-19 pandemic. COVID-19 is likely to have significantly accelerated the spread of AMR as the use of antibiotics has skyrocketed in the last 18 months with some reports describing indiscriminate prescription of broad spectrum antibiotics to all hospitalized patients.

Despite this urgency, there are currently only 43 antibiotics in clinical trials, with a minority representing new compound classes, none of which are effective against critical Gram-negative pathogens. In comparison, there are currently over 1000 compounds in clinical trials as cancer treatments and over 5000 clinical trials have been initiated for potential COVID-19 treatment since 2020.

Why Metals?
The vast majority of drugs today are purely organic molecules consisting of elements that make up less than 10% of the periodic table. Transition metals can form coordination compounds with organic ligands, forming highly diverse three-dimensional structures, exceeding the covalent limitations of a typical carbon atom. The use of metal complexes as drugs was initiated by the discovery and application of the anticancer drug cisplatin in 1978, with analogues still used in the majority of chemotherapeutic treatments today. Since then, several human clinical trials have been conducted with drugs containing metals such as titanium, iron, copper, gallium, ruthenium, palladium, silver, gold, or bismuth (with several more in development), proving that metal complexes are viable as non-cytotoxic drug candidates. One of the unique characteristics of metal complex drugs is their access to many different and unique modes of actions, ranging from redox reactions, generation of reactive oxygen or catalytic generation of other active species, ligand exchange or triggered ligand release, to general competitive or covalent inhibition of enzymes or binding to proteins. Many of these are difficult or even impossible to achieve with organic compounds alone.

Metalloantibiotics
In recent studies we have established that metal complexes are potentially better candidates for antibiotics than purely organic compounds. A comparative analysis of a library containing antimicrobial data on 300'000 compounds, including ~1000 metal complexes showed that the latter had a 10x higher hit-rate. At the same time we could show that the metal complexes did not display higher in vitro toxicity rates compared to organic molecules, contradicting the notion of general metal toxicity.

Since then we have started to investigate several metal complex classes more closely, gathering more data on their activity against a broader panel of drug-resistant bacteria, as well as preliminary in vivo data. In our current work we are both exploring new metal complexes for their antimicrobial potential as well as investigating the Mode of Action of our lead compounds to guide further development.

Antimicrobial Photodynamic Therapy (aPDT)
Almost all antibiotics are optimised to hit a single molecular target within a bacterium and hit it effectively. While this makes them potent, this is also their Achilles’s heel, as it applies strong selective pressure for mutations that alter the target or remove the antibiotic from inside the bacterial cell. Photodynamic therapy (PDT) functions via the localized generation of reactive oxygen species (ROS) by a photosensitizer (PS) when it is irradiated with specific wavelengths of light. Precise targeting of the PS to diseased tissue ensures that the irradiation event delivers ROS to a tightly defined area, leading to promiscuous damage of multiple cellular targets. PDT is already successfully used in cancer therapy in clinics today. The application of these principles in the design of next-generation antimicrobial agents (antimicrobial PDT; aPDT) is a nascent research area with exciting potential.
We are working towards the preparation of targeted and efficient metal-based photosensitizers for aPDT against localized bacterial infections.

Machine Learning Guided Metal-Complex Space Exploration
Chemical space is incredibly vast. Estimates put the number of “drug-like” organic molecules at around 1060, while excluding all transition metals. It is easy to see that if one iterates all possible ligands over all possible metals the permutations are nearly endless. Given this daunting uncharted space of metal complexes, an efficient and strategically guided approach to its exploration is required. Machine Learning algorithms have enabled many advances in the fields of chemical exploration and medicine but have only sparingly been applied to metal complexes and barely at all in the field of inorganic medicinal chemistry. We aim to use state-of-the art machine learning algorithms together with newly developed representations for metal complexes to efficiently explore the metal complex space for antimicrobial activity.
Further Reading
Metals in Medicine
-
Metallodrugs are unique: opportunities and challenges of discovery and development
-
Classification of Metal-Based Drugs according to Their Mechanisms of Action
Metal-based Antimicrobials
aPDT
-
Antimicrobial Photodynamic Therapy: A New Paradigm in the Fight Against Infections
-
Antimicrobial photodynamic therapy – what we know and what we don’t
Machine Learning in Antibiotic Research
Publications
- D. R. Husbands, Ç. Özsan, A. welsh, R. J. Gammons, A. Frei ”High-throughput triazole-based combinatorial click chemistry for the synthesis and identification of functional metal complexes” Nat. Commun. 2025, DOI: 10.1038/s41467-025-67341-z

- M. Orsi, A. Frei ”ELECTRUM: an electron configuration-based universal metal fingerprint for transition metal compounds” Digit. Discov. 2025, DOI: 10.1039/D5DD00145E
- A. Welsh, D. Husbands, A. Frei ”High-Throughput Combinatorial Metal Complex Synthesis” Angew. Chem. 2025, DOI: 10.1002/anie.202420204
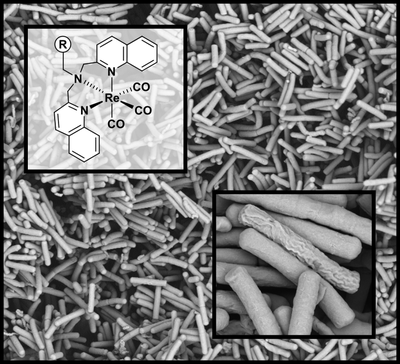
- S. Fulgencio, M. Scaccaglia, A. Frei ”Exploration of Rhenium Bisquinoline Tricarbonyl Complexes for their Antibacterial Properties” ChemBioChem 2024, DOI: 10.1002/cbic.202400435
- M. Scaccaglia, G. Pelosi, S. Pienilli, A. Frei ”Discovery of Antibacterial Manganese(I) Tricarbonyl Complexes through Combinatorial Chemistry” Chem. Sci. 2024, DOI: 10.1039/D3SC05326A

- M. Orsi, B. S. Loh, C. Weng, W. H. Ang, A. Frei ”Using Machine Learning to Predict the Antibacterial Activity of Ruthenium Complexes” Angew. Chem. 2024, DOI: 10.1002/anie.202317901

- A. van Niekerk, C. Joseph, A. Kavanagh, H. Dinh, A. J. Swarts, S. F. Mapolie, J. Zuegg, A. K. Cain, A. G. Elliott, M. A. T. Blaskovich, A. Frei ”The antimicrobial properties of Pd(II)- and Ru(II)-pyta complexes” ChemBioChem 2023, DOI: 10.1002/cbic.202300247

- A. Frei, A. D. Verderosa, A. G. Elliott, J. Zuegg, , M. A. T. Blaskovich ”Metals to combat antimicrobial resistance” Nat. Rev. Chem. 2023, DOI: 10.1038/s41570-023-00463-4

- A. Frei, A. G. Elliott, A. Kan, H. Dinh, S. Bräse, A. E. Bruce, M. R. Bruce, F. Chen, D. Humaidy, N. Jung, A. P. King, P. G. Lye, H. K. Maliszewska, A. M. Mansour, D. Matiadis, M. P. Muñoz, T.-Y. Pai, S. Pokhrel, P. J. Sadler, M. Sagnou, M. Taylor, J. J. Wilson, D. Woods, J. Zuegg, W. Meyer, A. K. Cain, M. A. Cooper, M. A. T. Blaskovich ”Metal Complexes as Antifungals? – From a Crowd-Sourced Compound Library to First In Vivo Experiments” JACS Au, 2022, DOI: 10.1021/jacsau.2c00308

- M. Krenn, Q. Ai, S. Barthel, N. Carson, A. Frei, N. C. Frey, P. Friederich, T. Gaudin, A. A. Gayle, K. M. Jablonka, R. F. Lameiro, D. Lemm, A. Lo, S. M. Moosavi, J. M. Nápoles-Duarte, A. K. Nigam, R. Pollice, K. Rajan, U. Schatzschneider, P. Schwaller, M. Skreta, B. Smit, F. Strieth-Kalthoff, C. Sun, G. Tom, G. F. von Rudorff, A. Wang, A. White, A. Young, R. Yu, A. Aspuru-Guzik “SELFIES and the future of molecular string representations” Patterns, 2022, DOI: 10.1016/j.patter.2022.100588
- S. M. Cooper, C. Siakalli, A. J. P. White, A. Frei, P. W. Miller, N. J. Long “Synthesis and anti-microbial activity of a new series of bis(diphosphine) rhenium(V) dioxo complexes”, Dalton Trans., 2022, DOI: 10.1039/D2DT02157A

- A. Frei, A. Rigby, . Thomas T. C. Yue, G. Firth, M. T. Ma, N. J. Long "To chelate thallium(I) – synthesis and evaluation of Kryptofix-based chelators for 201Tl", Dalton Trans., 2022, DOI: 10.1039/D2DT01074G

- J. L. Medina-Franco, E. López-López, E. Andrade, L. Ruiz=Azuara, A. Frei, D. Guan, J. Zuegg, M. A. T. Blaskovich "Bridging informatics and medicinal inorganic chemistry: Toward a database of metallodrugs and metallodrug candidates", Drug Discovery Today, 2022, DOI: 10.1016/j.drudis.2022.02.021
- D. V. Kama, A. Frei, M. Schuette-Smith, A. Brink, Chantel Swart, H. Braband, R. Alberto, A. Roodt “Exploring preliminary structural relationships and mitochondrial targeting of fac-[MI(CO)3]- bis(diarylphosphino)alkylamine complexes (M = 99Tc, Re)”, New J. Chem., 2021, DOI: 10.1039/D1NJ04273D
- A. Frei, S. Ramu, G. J. Lowe, H. Dinh, L. Semenec, A. G. Elliott, J. Zuegg, A. Deckers, N. Jung, S. Bräse, A. K. Cain, M. A. T. Blaskovich "Platinum Cyclooctadiene Complexes with Activity against Gram-positive Bacteria", ChemMedChem 2021, 16, 2021-2029. Front Cover DOI: 10.1002/cmdc.202100157

- D. V. Kama, A. Frei, A. Brink, H. Braband, R. Alberto, A. Roodt "New approach for the synthesis of water soluble fac-[MI(CO)3]+ bis(diarylphosphino)alkylamine complexes (M=99Tc, Re" Dalton Trans. 2021 DOI: 10.1039/D1DT03234H
- A. Frei, A. P. King, G. J. Lowe, A. K. Cain, F. L. Short, H. Dinh, A. G. Elliott, J. Zuegg, J. J. Wilson, M. A. T. Blaskovich "Nontoxic Cobalt(III) Schiff Base Complexes with Broad-Spectrum Antifungal Activity", Chemistry – A European Journal 2021, 27, 2021-2029. Cover Feature DOI: 10.1002/chem.202003545

- A. Notaro, A. Frei, R. Rubbiani, M. Jakubaszek, U. Basu, S. Koch, C. Mari, M. Dotou, O. Blacque, J. Gouyon, F. Bedioui, N. Rotthowe, R. F. Winter, B. Goud, S. Ferrari, M. Tharaud, M. Řezáčová, J. Humajová, P. Tomšík, G. Gasser "Ruthenium(II) Complex Containing a Redox-Active Semiquinonate Ligand as a Potential Chemotherapeutic Agent: From Synthesis to In Vivo Studies", Journal of Medicinal Chemistry 2020, 63, 5568-5584. DOI: 10.1021/acs.jmedchem.0c00431

- A. Frei, J. Zuegg, A. G. Elliott, M. Baker, S. Braese, C. Brown, F. Chen, C. G. Dowson, G. Dujardin, N. Jung, A. P. King, A. M. Mansour, M. Massi, J. Moat, H. A. Mohamed, A. K. Renfrew, P. J. Rutledge, P. J. Sadler, M. H. Todd, C. E. Willans, J. J. Wilson, M. A. Cooper, M. A. T. Blaskovich "Metal complexes as a promising source for new antibiotics", Chemical Science 2020, 11, 2627-2639. Inside Cover DOI: 10.1039/C9SC06460E

- A. Frei "Metal Complexes, an Untapped Source of Antibiotic Potential", Antibiotics 2020, 9, 90. DOI: 10.3390/antibiotics9020090

- A. Frei, M. Amado, M. A. Cooper, M. A. T. Blaskovich "Light-Activated Rhenium Complexes with Dual Mode of Action against Bacteria", Chem. – Eur. J. 2019, 26, 2852-2858. DOI: 10.1002/chem.201904689

- A. Frei, E. Fischer, B. C. Childs, J. P. Holland, R. Alberto "Two is better than one: difunctional high-affinity PSMA probes based on a [CpM(CO)3] (M = Re/99mTc) scaffold", Dalton Trans. 2019, 48, 14600-14605. DOI: 10.1039/C9DT02506E

- A. Frei "Synthetic Routes towards Multifunctional Cyclopentadienes", Chemistry – A European Journal 2019, 25, 7074-7090. DOI: 10.1002/chem.201900276

- R. Bolliger, A. Frei, H. Braband, G. Meola, B. Spingler, R. Alberto "Chemistry at High Dilution: Dinuclear 99mTc Complexes", Chemistry – A European Journal 2019, 25, 7101-7104. DOI: 10.1002/chem.201901161

- P. P. Mokolokolo, A. Frei, M. S. Tsosane, D. V. Kama, M. Schutte-Smith, A. Brink, H. G. Visser, G. Meola, R. Alberto, A. Roodt "Nuclearity manipulation in Schiff-base fac-tricarbonyl complexes of Mn(I) and Re(I)", Inorganica Chimica Acta 2018, 471, 249-256. DOI: 10.1016/j.ica.2017.10.036

- A. Frei, B. Spingler, R. Alberto "Multifunctional Cyclopentadienes as a Scaffold for Combinatorial Bioorganometallics in [(η5-C5H2R1R2R3)M(CO)3] (M=Re, 99mTc) Piano-Stool Complexes", Chem. – Eur. J 2019, 24, 10156-10164. DOI: 10.1002/chem.201801271

- A. Frei, P. P. Mokolokolo, R. Bolliger, H. Braband, M. S. Tsosane, A. Brink, A. Roodt, R. Alberto "Self-Assembled Multinuclear Complexes Incorporating 99mTc", Chemistry – A European Journal 2018, 24, 10397-10402. DOI: 10.1002/chem.201800600

- J. P. Kraack, A. Frei, R. Alberto, P. Hamm "Ultrafast Vibrational Energy Transfer in Catalytic Monolayers at Solid–Liquid Interfaces", The Journal of Physical Chemistry Letters 2017, 8, 2489-2495. DOI: 10.1021/acs.jpclett.7b01034

- A. Frei, D. Sidler, P. Mokolokolo, H. Braband, T. Fox, B. Spingler, A. Roodt, R. Alberto "Kinetics and Mechanism of CO Exchange in fac-[MBr2(solvent)(CO)3]− (M = Re, 99Tc)", Inorganic Chemistry 2016, 55, 9352-9360. DOI: 10.1021/acs.inorgchem.6b01503

- A. Frei, R. Rubbiani, S. Tubafard, O. Blacque, P. Anstaett, A. Felgenträger, T. Maisch, L. Spiccia, G. Gasser "Synthesis, Characterization, and Biological Evaluation of New Ru(II) Polypyridyl Photosensitizers for Photodynamic Therapy", Journal of Medicinal Chemistry 2014, 57, 7280-7292. DOI: 10.1021/jm500566f

- A. Leonidova, T. Joshi, D. Nipkow, A. Frei, J.-E. Penner, S. Konatschnig, M. Patra, G. Gasser "An Environmentally Benign and Cost-Effective Synthesis of Aminoferrocene and Aminoruthenocene", Organometallics 2013, 32, 2037-2040. DOI: 10.1021/om400009g







